There is no equilibrium
The chemical equilibrium is a very fugitive state. Both Kinetics and Thermodyanmics try to describe it

Most reactions that happen in nature are part of a chemical equilibrium, which means we have a forward and backward reaction. In the Industry one example is the Haber-Bosch-Verfahren, where Nitrogen and Hydrogen are forced to become Ammonia.
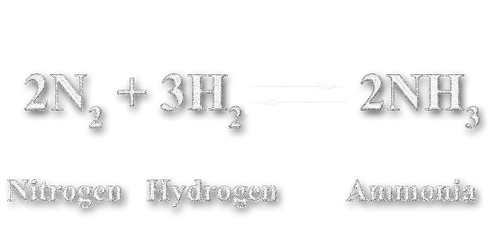
The question is: How does this two sided arrow work? What is an equilibrium? And what can we do to create Ammonia? This crazy arrow let's us know, that at any time in this process Ammonia is being produced and at the same time it dissolutes back into Nitrogen and Hydrogen. Both reactions are always happening.
And this is where the problem really starts. If both processes happen simultaneous, how can there be equilibrium? The answer for this may be found in the principles of thermodynamics. Every reaction is defined by the change in its free Gibbs Enthalpy ΔG. Gibbs defined one of the four forms of energy we know in thermodynamics, the other three being the inner Energy U, the Helmholtz free Energy A and the Enthalpy H. All of these are used to describe Systems and Reactions.
Going on we'll need ΔG for describing our reaction, where ΔG is the change in Gibbs free Enthalpy. If ΔG is smaller than 0, the reactions is spontaneous and moves towards products, in Chemistry this is called exergonic. As mentioned above this would be Ammonia. If it is greater than 0 it is endergonic and we would receive Nitrogen and Hydrogen. For equilibrium we need to achieve a ΔG-Value of ZERO, not 0.1 or such. We need absolute zero.
ΔG is the sum of chemical potentials on each side of the equation. The chemical potential is the partial, molare Derivate of the Gibbs Free Enthalpy in mathematical Terms. More simpler spoken it is the potential that one substance inherits. Now lets describe it a bit simpler. We have hot and cold items in our reaction and all of them want to achieve room temperatur. ΔG is the temperature we are going to achieve, while the chemical potential of our different molecules are either far away from room temperature or very close.
So what we truly need to do, to achieve equilibrium, is to keep everything as constant as possible - but even then we will only achieve an equilibrium on a macroscopic level. On a microscopic level there may ever be movement.
Thermodynamics take us only so far. Kinetics may help interpret at which point we achieve the equilibrium. Because the forward reaction and the backward reaction happen at different speeds dependent on their concentrations. So at one point we may achieve, that they both happen at the same speed. This could be called equilibrium. But it is also a fugitive state.
So at the end, both thermodynamics and kinetics don't speak of an absolute state, where the reaction dies and equilibrium is achieved. It is an ever dynamic process. Since Le Chatelier we know that temperature, pressure and the adding of reactants may influence this fugitive state greatly. So next time you think about equilibrium, keep in mind that it is no static state, but a dynamic process - in chemistry as well as in life.

